Ch. 1 Structure of the Atom
Return to TOC
Atomic Discoveries Summarized
- Antoine Lavoisier: law of conservation of matter
(1774)
- Joseph Proust: law of constant composition (1799)
- John Dalton: atomic theory (1803-1808)
- law of multiple proportions
- Michael Faraday: electric current can cause chemical reactions (1834)
- Sir William Crooks: cathode ray tube (1870s)
- J.J. Thomson: charge to mass ratio (1897)
- Robert Millikan: oil drop experiment (1909)
- Ernest Rutherford: gold foil experiment
- nuclear model
- proton (1919)
- Niels Bohr: solar system model (1913)
- Erwin Schrödinger: wave-mechanical theory (1927)
- Werner Heisenberg: uncertainty principle (1920s)
| Name |
Symbol |
Charge (Coulombs) |
Mass |
| Electron |
e or e−
|
−1.602⋅10−19
(-1) |
9.109⋅10−28
(5.486⋅10−4)
|
| Proton |
p |
1.602⋅10−19
(+1) |
1.673⋅10−24
(1.0073) |
| Neutron |
n |
0 |
1.675⋅10−25
(1.0087) |
Electromagnetic Spectrum
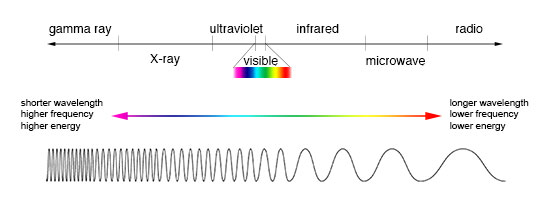
Light
(Wavelength)(Frequency)=(Speed of
Light)
λv=c=299,792,458 m/s≈3.0⋅108 m/s
(Energy)=(Planck's
constant)(Frequency)
E=hv=hλc=mc2,h=6.63⋅10−34 J⋅s
Example Problem
Find the frequency and energy of 400. nm light
λ=400. nm
(400. nm)v=3.0⋅108 m s−1
v=4.00⋅10−7 m3.0⋅108 m s−1=7.5⋅1014 s−1
E=hv=(6.63⋅10−34 J s)(7.5⋅1014 s−1)=5.0⋅10−19 J
Energy in a orbital (principal quantum number) n: En=n2h2−2π2me4=n2−2.178⋅10−18 J
Energy Levels
Principal Energy Levels (Shells): 1, 2, 3, 4,...
Sublevels
(Subshells): s, p, d, f
Orbitals: 1, 3, 5, 7 pairs of e−
can fit in sublevel
Electrons
Electron Configuration: e.g. 1s22s22p63s23p64s23d10… or abbreviated
Electron Configuration for Fe
Fe=1s22s22p63s23p64s23d6
Fe=[Ar]4s23d6
Valence Electrons: Outside electrons; sum of s and p
orbitals
Hund's Rule: p, d, and f orbitals must have
one electron in each orbital before they can pair up
